 In our dynamic world, a better syndromic Point-of-Care testing is key to improve linkage to care. It enables swift diagnosis, timely treatment, and enhanced patient outcomes. Syndromic screening is a vital tool in controlling infectious diseases, reducing spread, and preventing outbreaks. However, it is often limited to lab settings and rarely available at the Point-of-Care. Beyond patient convenience, it optimizes resource use, ensuring healthcare remains accessible and fair to all.
In our dynamic world, a better syndromic Point-of-Care testing is key to improve linkage to care. It enables swift diagnosis, timely treatment, and enhanced patient outcomes. Syndromic screening is a vital tool in controlling infectious diseases, reducing spread, and preventing outbreaks. However, it is often limited to lab settings and rarely available at the Point-of-Care. Beyond patient convenience, it optimizes resource use, ensuring healthcare remains accessible and fair to all.
MagIA diagnostics, driven by these ideals, is pioneering an innovative on-the-spot system, the M-for-Screen. It is fusing an easy-to-use cartridge approach with an easy-to-deploy analyzer, all dedicated to raising the bar at the point of need.
MagIA diagnostics innovative diagnostic technology is the culmination of a remarkable 15-year collaboration among several research institutes based in Grenoble, France. Its innovation, protected by multiple patents, employs magnetic interactions at the submillimeter scale for pathogen detection. The developed immunoassay has been extensively studied in various studies, unveiling its versatility across various biological parameters, its substantial clinical potential, and its suitability for industrial applications. The company itself was created in 2017, with the ambition to make a deeptech breakthrough available to as many people as possible and have a major impact on the most serious public health issues.
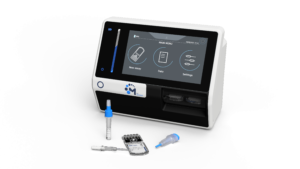
The first product, the MagIA IBC test, has been designed for the simultaneous detection of HIV, Hepatitis B & C infections from capillary blood. This concerns approximately 400 million individuals worldwide, currently infected with HIV and Viral Hepatitis. Whereas HIV is regularly tested on the spot, viral hepatitis testing is usually not included, and awareness of viral Hepatitis status remains low, with less than 20% of those infected being aware of their status. The MagIA IBC test would answer the need for a combined screening approach at the Point-of-Care to better manage epidemics and link key populations to care.
Benkei has helped MagIA diagnostics to promote its technological innovations by implementing a scientific publication strategy. This strategy was defined to meet the need for scientific recognition by peers, scientific credibility in relation to prospects and customers, investors and competitors, but also to highlight historical and/or renowned partners. In practice, Benkei set up a roadmap, identified the type of publications (academic, commercial, application), helped to choose the most suitable journals, and assisted MagIA in constructing the framework and messaging of 2 publications, then in managing their editorial process.